Advanced hemodynamic variables
Afterload
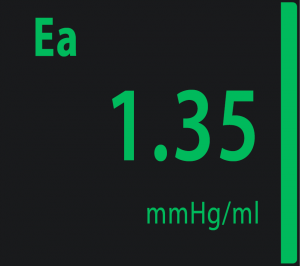
Ea – Arterial Elastance
 |
The left ventricle ejects blood into the aorta and arterial system. The arterial load represents the force against which the left ventricle must eject. This “arterial load” contains the resistances to flow and the elasticity of arterial system. Effective arterial elastance (Ea) is mainly related to the elasticity of the arterial system. The elasticity of the large arteries allows these vessels to expand during the ejection phase accommodating more blood and then, because of the systolic stored of elastic energy relapsed during diastole, assures diastolic blood flow in periphery. |
This arterial property allows to convert pulsatile flow in more continuous one according to the organ vascular resistances. The more rigid the vessels, the lower the volume (i.e. higher Ea), the less rigid the vessels, the higher the volume (i.e. lower Ea).
Arterial elastance is physiologically determined by the ratio of end systolic pressure and stroke volume (ESP/SV, mmHg/mL). ESP corresponds to aortic valve closure which is the dicrotic pressure (Dic) on the pressure wave. In MostCareUp ESP is replaced by the dicrotic pressure (Dic).
Ea = Dic / SV
Physiological range at rest: from 1.10 to 1.40 mmHg/mL
Arterial elastance can change according to different drug administered (e.g. Ca++ antagonist, B-blocker, nitrates, volume-induced vasodilation, etc…).
1.Nichols WW, O’Rourke MF. Vascular impedance. In: McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. 4th ed. London, UK: Edward Arnold; 1998:54–97, 243–283, 347–395.
2.Chantler PD, Lakatta EG, Najjar SS. Arterial-ventricular coupling: mechanistic insights into cardiovascular performance at rest and during exercise. J Appl Physiol (1985). 2008 Oct;105(4):1342-51.
3.Scolletta S, Bodson L, Donadello K, Taccone FS, Devigili A, Vincent JL, De Backer D. Assessment of left ventricular function by pulse wave analysis in critically ill patients. Intensive Care Med 2013;39: 1025-33.
4.Morelli A, Singer M, Ranieri VM, D’Egidio A, Mascia L, Orecchioni A, Piscioneri F, Guarracino F, Greco E, Peruzzi M, Biondi-Zoccai G, Frati G, Romano SM. Heart rate reduction with esmolol is associated with improved arterial elastance in patients with septic shock: a prospective observational study. Intensive Care Med 2016.
5.Messina A, Romano SM, Bonicolini E, Colombo D, Cammarota G, Chiostri M, Della Corte F, Navalesi P, Payen D, Romagnoli S. Cardiac cycle efficiency and dicrotic pressure variations: new parameters for fluid therapy: A pilot observational study. Eur J Anaesthesiol 2017; 34:1–9.
6.Monge Garcia MI, Jian Z, Settels JJ, Hatib F, Cecconi M Pinsky MR. Reliability of effective arterial elastance using peripheral arterial pressure as surrogate for left ventricular end-systolic pressure. J Clin Monit Comput. 2018 Dec 14.
PPV/SVV – Dynamic Elastance
 |
Dynamic elastance, is the ratio between PPV and SVV. It has been suggested as a variable that represents the dynamic changes of arterial load and tone in mechanically ventilated patients. MostCareUp calculates the averaged value of PPV/SVV in a window of 15 seconds (default). |
Physiological range at rest: from 0.5 to 1.5 units.
Dynamic elastance can change according to different clinical settings (e.g. hypotensive patients, vasodilation) and may be useful for the prediction of blood pressure response to fluid challenge or vasoactive drugs administration.
1.Pinsky MR (2002) Functional hemodynamic monitoring: applied physiology at the bedside. Springer, Berlin, pp 537–552 book.
2.Pinsky MR. Probing the limits of arterial pulse contour analysis to predict preload responsiveness. Anesth Analg. 2003 May;96(5):1245-7.
3.Monge García MI, Gil Cano A, Gracia Romero M. Dynamic arterial elastance to predict arterial pressure response to volume loading in preload-dependent patients. Crit Care. 2011;15(1):R15.
4.García MI, Romero MG, Cano AG, Aya HD, Rhodes A, Grounds RM, Cecconi M.Dynamic arterial elastance as a predictor of arterial pressure response to fluid administration: a validation study. Crit Care. 2014 Nov 19;18(6):626.
5.Guinot PG, Bernard E, Levrard M, Dupont H, Lorne E. Dynamic arterial elastance predicts mean arterial pressure decrease associated with decreasing norepinephrine dosage in septic shock. Crit Care. 2015 Jan 19;19:14.
Left ventricular systolic function
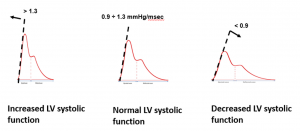
dP/dtmax – Maximum slope of the systolic upstroke
 |
dP/dtmax is the maximum slope of arterial blood pressure during systolic upstroke. Physiological range at rest: 0.90 to 1.30 mmHg/msec |
Because MostCareUp measures dP/dtmax at peripheral sites, changes in arterial elastance and systemic vascular resistance may influence dP/dtmax value.
dP/dtmax can change according to different clinical settings (e.g. congestive heart failure, haemorrhagic shock, deep sedation and vasodilation) and may be useful for assessing the response of left ventricle to inotropes and vasoactive medications.
dP/dtmax values higher than 1.7 mmHg/msec is unphysiological and might suggest that the arterial waveform is underdamped
1.De Hert SG, Robert D, Cromheecke S, Michard F, Nijs J, Rodrigus IE. Evaluation of left ventricular function in anesthetized patients using femoral artery dP/dt(max). J Cardiothorac Vasc Anesth. 2006 Jun;20(3):325-30.
2.Tartiere JM, Logeart D, Beauvais F, Chavelas C, Kesri L, Tabet JY, et al. Noninvasive radial pulse wave assessment for the evaluation of left ventricular systolic performance in heart failure. Eur J Heart Fail. 2007;9(5):477–83
3.Tartière JM, Tabet JY, Logeart D, Tartière-Kesri L, Beauvais F, Chavelas C, Cohen Solal A. Noninvasively determined radial dP/dt is a predictor of mortality in patients with heart failure. Am Heart J. 2008 Apr;155(4):758-63.
4.Chew MS, Åneman A. Haemodynamic monitoring using arterial waveform analysis. Curr Opin Crit Care. 2013 Jun;19(3):234-41.
5.Scolletta S et al. Assessment of left ventricular function by pulse wave analysis in critically ill patients. Intensive Care Med 2013;39: 1025-33.
6.Monge Garcia MI, Jian Z, Settels JJ, Hunley C, Cecconi M, Hatib F, Pinsky MR. Performance comparison of ventricular and arterial dP/dtmax for assessing left ventricular systolic function during different experimental loading and contractile conditions. Crit Care. 2018 Nov 29;22(1):325.
V-A coupling
Dic – Dicrotic pressure
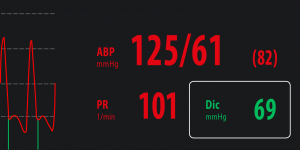 |
Dicrotic pressure is the pressure at the end of systolic phase which correspond to the closure of aortic valve. MostCareUp, based on a sophisticated algorithm working at 1000 samples per seconds, can detect beat by beat the value of dicrotic pressure, marking the position of dicrotic notch by a vertical green line. |
Dicrotic pressure provides information on the relationship between systolic function and the characteristics of the arterial tree (V-A coupling) and its value is accepted to be physiologically close to the MAP value.
Dicrotic pressure is often estimated as the central systolic blood pressure multiplied by 0.9, while MostCareUp measures Dic real-time at each cardiac cycle.
Physiological range: 70 – 105 mmHg.
Dic may change according to different clinical settings (e.g. tachycardia, vasoconstriction, sepsis) and may be useful for evaluating the response of the cardiovascular system to cardio-vasoactive drugs.
1.Lewis T. The factors influencing the prominence of the dicrotic wave. J Physiol 1906; 34:414–429.
2.Smith D, Craige E. Mechanism of the dicrotic pulse. Br Heart J 1986; 56:531–534.
3.Nichols WW, O’Rourke MF 1990: McDonald’s blood flow in arteries, third edition. Sevenoaks: Edward Arnold.
4.Chantler PD, Lakatta EG, Najjar SS. Arterial-ventricular coupling: mechanistic insights into cardiovascular performance at rest and during exercise. J Appl Physiol (1985). 2008 Oct;105(4):1342-51.
5.Pavoni V, Romagnoli S, Batignani G, Gianesello L, Horton A, Romano SM. Unsuspected Heart Failure: Usefulness of a Minimally Invasive Hemodynamic Monitoring System. J Anesth Clin Res 2012; 3(8).
6.Scolletta S et al. Assessment of left ventricular function by pulse wave analysis in critically ill patients. Intensive Care Med 2013;39: 1025-33.
7.Guarracino F, Ferro B, Morelli A, Bertini P, Baldassarri R, Pinsky MR. Ventriculoarterial decoupling in human septic shock. Crit Care. 2014 Apr 24;18(2):R80.
8.Messina A, Romano SM, Bonicolini E, Colombo D, Cammarota G, Chiostri M, Della Corte F, Navalesi P, Payen D, Romagnoli S. Cardiac cycle efficiency and dicrotic pressure variations: new parameters for fluid therapy: A pilot observational study. Eur J Anaesthesiol 2017; 34:1–9.
9.Ristalli F, Romano SM, Stolcova M, Meucci F, Squillantini G, Valente S, Di Mario C. Hemodynamic monitoring by pulse contour analysis during trans-catheter aortic valve replacement: A fast and easy method to optimize procedure results. Cardiovasc Revasc Med 2018 Jul 19.
CCE – Cardiac Cycle Efficiency
 |
Cardiac Cycle Efficiency describes hemodynamic performance in term of energy expenditure. Indeed, CCE depends on the energy required to generate a given SV, which depends on the interaction between pump function and arterial system (i.e. A-V coupling). See more details in the Efficiency paragraph. |
Efficiency
CPO – Cardiac Power Output
 |
Cardiac power output represents cardiac pumping ability to generate blood flow. It expresses the cardiac power reserves that can be recruited to increase the heart’s pumping ability. The higher the CPO, the higher the recruitable reserves to increase pumping ability. Physiological range at rest: from 0.80 to 1.20 Watt. |
CPO has been demonstrated to be a good predictor for mortality in heart failure patients.
Available also:
CPI = MAP · CI/ k cardiac power index from 0.50 to 0.70 W/m2
1.Cotter G, Williams SG, Vered Z, Tan LB. Role of cardiac power in heart failure. Curr Opin Cardiol. 2003 May;18(3):215-22.
2.Fincke R, Hochman JS, Lowe AM et al (2004) Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: a report from the SHOCK trial registry. J Am Coll Cardiol 44:340–348.
3.Mendoza DD, Cooper HA, Panza JA. Cardiac power output predicts mortality across a broad spectrum of patients with acute cardiac disease. Am Heart J. 2007 Mar;153(3):366-70.
4.Hothi SS, Tan LB, Cotter G. Resting cardiac power index and prediction of prognosis in heart failure. Eur J Heart Fail. 2015 Jul;17(7):642-4.
5.Nathania M, Hollingsworth KG, Bates M, Eggett C, Trenell MI, Velicki L, Seferovic PM, MacGowan GA, Turnbull DM, Jakovljevic DG. Impact of age on the association between cardiac high-energy phosphate metabolism and cardiac power in women. Heart. 2018 Jan;104(2):111-118.
CCE – Cardia Cycle Efficiency
 |
Cardiac Cycle Efficiency describes hemodynamic performance in term of energy expenditure. Indeed, CCE depends on the energy required to generate a given SV, which depends on the interaction between pump function and arterial system (i.e. A-V coupling). Many factors may influence CCE, for example changes in left ventricular function, heart rate, preload, afterload, including arterial elastance and reflected pressure waves. |
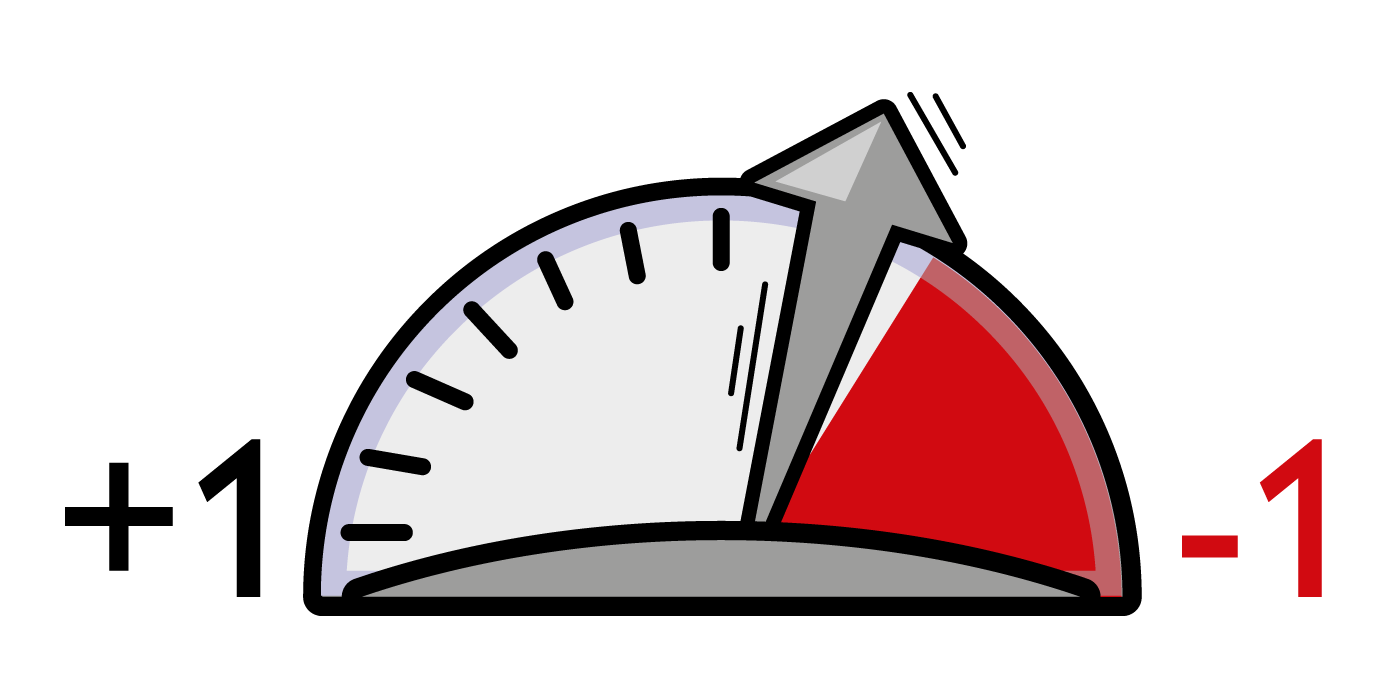
The value of CCE can range from +1 (ideal condition with no energy expenditure) to negative values: the greater the energy expenditure to generate a given SV, the lower the CCE value.
|
CCE is computed beat by beat as the ratio of the sum of systolic powers [W(t)sys] to the sum of all powers of entire cycle [W(t)beat]. Physiological range: -0.2 – 0.3 units. |
 |
CCE can change according to different clinical settings (e.g. bradycardia, tachycardia, poor myocardial contractility, increased or reduced venous return, changes of systemic vascular resistance) and may be useful for assessing the hemodynamic response to cardioactive and vasoactive drugs. Additionally, monitoring the trend of CCE can be of value in preventing unexpected hemodynamic impairments and may help in clinical decision-making.
1.Giglioli C, Landi D, Cecchi E, Chiostri M, Gensini GF, Valente S, Ciaccheri M, Castelli G, Romano SM. Effects of ULTRAfiltration vs DIureticS on clinical neurohormonal and hemodynamic variables in patients with deCOmpensated heart failure: the ULTRADISCO study. Eur J Heart Fail 2011;13(3): 337-46.
2.Pavoni V, Romagnoli S, Batignani G, Gianesello L, Horton A, Romano SM. Unsuspected Heart Failure: Usefulness of a Minimally Invasive Hemodynamic Monitoring System. J Anesth Clin Res 2012; 3(8).
3.Romano SM. Cardiac cycle efficiency: a new parameter able to fully evaluate the dynamic interplay of the cardiovascular system. Int J Cardiol 2012; 155:326-7
4.Onorati F, Santini F, Amoncelli E, Campanella F, Chiominto B, Faggian G, Mazzucco A. How should I wean my next intra-aortic balloon pump? Differences between progressive volume weaning and rate weaning. J Thorac Cardiovasc Surg 2012;145: 1214-21.
5.Scolletta S et al. Assessment of left ventricular function by pulse wave analysis in critically ill patients. Intensive Care Med 2013;39: 1025-33.
6.Morelli A, Singer M, Ranieri VM, D’Egidio A, Mascia L, Orecchioni A, Piscioneri F, Guarracino F, Greco E, Peruzzi M, Biondi-Zoccai G, Frati G, Romano SM. Heart rate reduction with esmolol is associated with improved arterial elastance in patients with septic shock: a prospective observational study. Intensive Care Med 2016.
7.Messina A, Romano SM, Bonicolini E, Colombo D, Cammarota G, Chiostri M, Della Corte F, Navalesi P, Payen D, Romagnoli S. Cardiac cycle efficiency and dicrotic pressure variations: new parameters for fluid therapy: A pilot observational study. Eur J Anaesthesiol 2017; 34:1–9.
8.Ristalli F, Romano SM, Stolcova M, Meucci F, Squillantini G, Valente S, Di Mario C. Hemodynamic monitoring by pulse contour analysis during trans-catheter aortic valve replacement: A fast and easy method to optimize procedure results. Cardiovasc Revasc Med 2018 Jul 19.

